Background
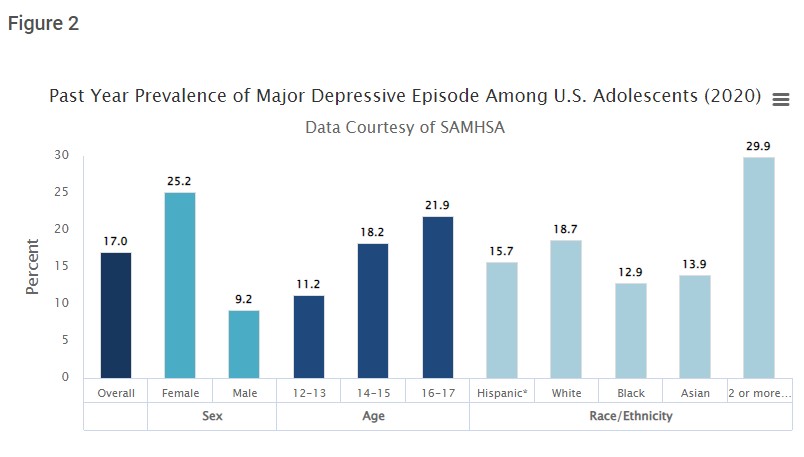
Depression and other mental illnesses are major problems in society. The cost of mental illnesses in the United States alone is closing in on $200 billion USD. Even with an enormous cost, many individuals are not receiving adequate assistance or reaching out to professionals, in part due to social norms and cost. Although there are many adults in the United States with depression, it may be relatively novel that these disorders stem from the adolescence period, and not necessarily the immediate time frame. The reason the adolescence periods are so crucial is that the brain and body are changing quite rapidly, and changes in hormones, growth factors, and nutrient intake all impact the brain. In turn, the brain can control the release of certain hormones as well. These feedback loops are crucial when examining the period of adolescence, which should be defined in this scenario as age 10 – 23 years old, partially due to the flexibility of the body at this age.
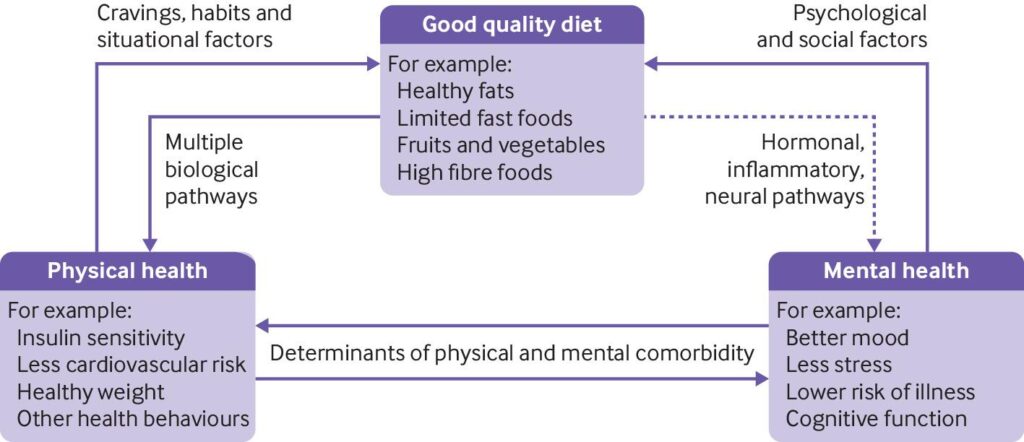
A major environmental factor for proper development is nutrition. Evidence in the literature suggests that too steep of a calorie deficit can have negative impacts on long-term brain health. The literature also suggests that over-consumption of calories during adolescence is just as harmful, causing obesity, early onset diabetes, and inflammation. Until recently, the impact of a specific diet has not been known to impact brain structure. This alteration in brain structure can ultimately change behavior and increase the likelihood of depression and other mental illnesses.
Journal Review
The aim of this article is to demonstrate the impact of a high-fat diet on brain structure and composition. Firstly, mice were taken at 2 months old and fed either a standard diet (SD) or a high-fat diet (HFD). After examining both diets’ compositions, it seems as if the HFD is calorically dense than the SD which may impact the results of this paper. This difference in calories had a significant impact on weight gain, with the HFD group gaining significantly more weight over the course of a month. With that being said, the difference in calories may or may not be significant, the researchers should have included this analysis in the research. Also, the conclusion from this paper should include the comparison between a high-calorie high-fat diet compared to a standard diet rather than a HFD versus a standard diet.
It is also important to note that the brain is not composed of only neurons, there are many other cells that make up the brain. These cells have an important role in inflammation, protection, regulation, and metabolism. One of these cells is called astrocytes, a specialized glial cell involved in providing building blocks to neurons. These astrocytes are also involved in protecting neurons by providing glutathione precursors to neurons, with glutathione being the body’s main antioxidant. Although these astrocytes play a large role in many physiological processes, under specific environmental or genetic conditions, they can lead to pathophysiological disease states. This principle goes for many cells that have a role in regulating the immune system such as the different macrophage phenotypes, M1 and M2.
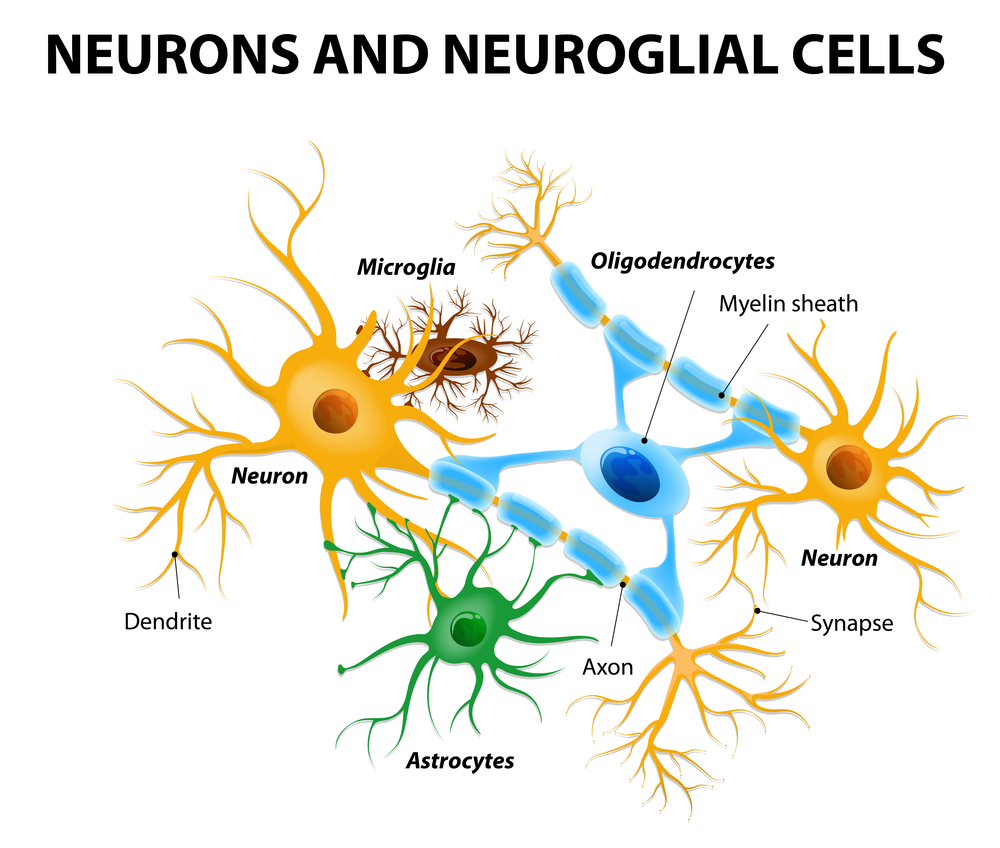
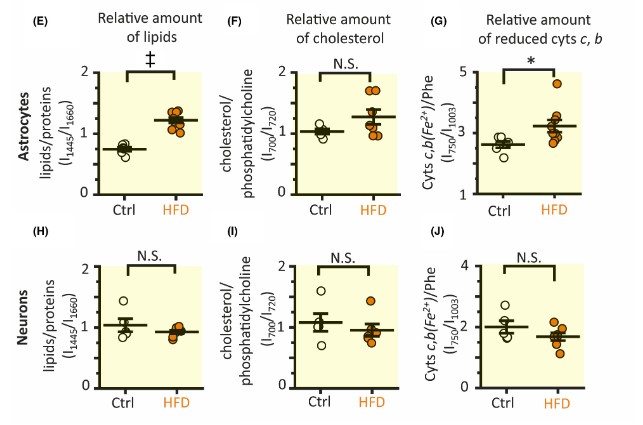
The importance of these astrocytes regarding dietary intake of fuel sources is regarding the mitochondria. The mitochondria house fuel from glycolysis, and use it to power the Krebs cycle, and electron transport chain, ultimately leading to the production of ATP. Since the cell can control the expression of genes through epigenetic modifications, it is not surprising that after time a change in diet changes the expression of metabolic genes. For example, a diet rich in carbohydrates may have higher expression of glycolysis genes, while a diet rich in fats may have higher expression of lipid utilization genes. In relevance to this journal article, the researchers found that the astrocytes in the hippocampus of the HFD mice had significantly higher amounts of lipids. The researchers also found higher amounts of c- and b-type cytochromes in the astrocytes of the HFD mice. However, when analyzing neurons, the HFD mice had no significant changes in lipid or cytochrome amount. From this data, the researchers concluded that a HFD induces metabolic changes in astrocytes not neurons in the hippocampus, which may affect the production of reactive oxygen species (ROS) present in the brain.
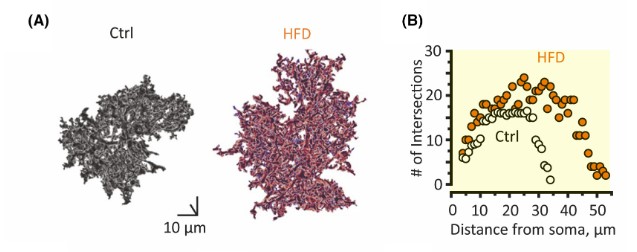
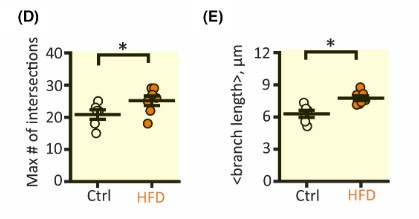
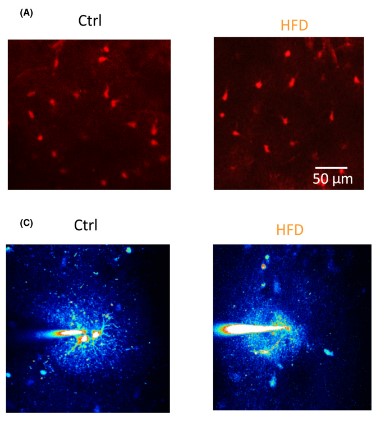
Another aspect of astrocyte biology the researchers examined was morphology. To examine the size of astrocytes, hippocampal astrocytes were extracted and stained with dyes. After dying the components, a 3-D model can be constructed. After construction, the researchers concluded that astrocyte size in the hippocampus of HFD mice had increased branch length and domain area, which impacts the overall area that the astrocyte has action over. Next, the researchers used fluorescence to visualize the number of cells and the number of coupled cells in the hippocampus. The resulting images display that the HFD did not significantly affect the total number of cells nor the number of coupled cells in the hippocampus.
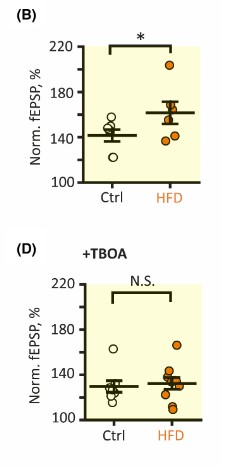
The researchers then focused on long-term potentiation (LTP), a process that strengthens the connection between synapses such that a long-term increase in neurotransmission occurs. It was found that long-term potentiation magnitude was significantly higher in HFD mice cells compared to control cells. The researchers then blocked the glutamate transporters using, TFB-TBOA, and repeated the previous experiment and found that field excitatory postsynaptic potentials (fEPSPs) magnitude, a measurement of LTP was no longer significantly different in HFD cells when compared to control cells. The result of this study is that the enhanced glutamate transport via HFD astrocytes may lead to enhanced LTP in the hippocampus.
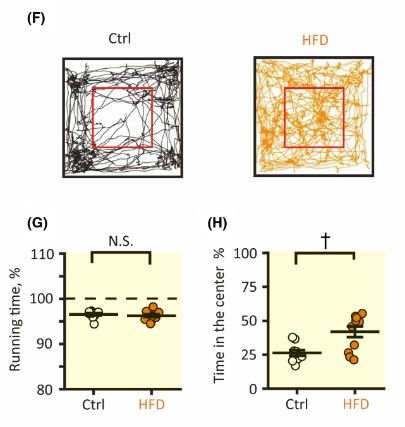
To determine the impact of a HFD on behavior, the researchers performed an open-field test. The mice fed a HFD spent an equal amount of time moving, but a significantly higher amount of time in the center of the arena. Being in the center of the test indicates a lessening of anxiety. Therefore, the researchers concluded that a HFD led to an increase in synaptic potentiation in the hippocampus. This increase in hippocampal long-term potentiation led to a decrease in anxiety compared to mice fed a standard diet.
Takeaways
There are several takeaways from this paper, including the possibility that a short-term increase in fat intake may have a beneficial impact on astrocyte physiology. These benefits may include decreasing anxiety through an increase in synaptic strengthening in the hippocampus. This study is a very fundamental paper and for the data to be reliably relayed to the public, the experiments need to be reproduced in a variety of environments.
Critiques
Keeping in mind that this study is a very basic paper, involving only a few data points in a specific brain region, the paper still has flaws that should be fixed in future studies. First, the caloric value of each diet should be properly gathered and used as a control, this way caloric variability does not influence the results. Secondly, more anxiety tests need to be run to make the conclusions the researchers make. The researchers use an open-field test to determine the impact of diet on anxiety, however, several tests should have been conducted to reproduce the results.

Meet The Author
Hello everyone,
My name is Joshua Giblin. I am a post-bachelor researcher/research technician at USC. My interests range from nutrition to nanomedicine and also practical science to improve everyday life. Through this blog, I aim to communicate practical scientific research and present it to curious individuals so that an educated decision can be made. Thank you for reading the blog and showing your support.
Literature cited
- Firth, J., Gangwisch, J. E., Borsini, A., Wootton, R. E., & Mayer, E. A. (2020). Food and mood: How do diet and nutrition affect mental wellbeing? BMJ, 369, m2382. https://doi.org/10.1136/bmj.m2382
- Fu, W., & Jhamandas, J. H. (2020). Chapter 20—Amylin and amylin receptors in Alzheimer’s disease. In C. R. Martin & V. R. Preedy (Eds.), Genetics, Neurology, Behavior, and Diet in Dementia (pp. 309–324). Academic Press. https://doi.org/10.1016/B978-0-12-815868-5.00020-7
- Jha, M. K., Kim, J.-H., Song, G. J., Lee, W.-H., Lee, I.-K., Lee, H.-W., An, S. S. A., Kim, S., & Suk, K. (2018). Functional dissection of astrocyte-secreted proteins: Implications in brain health and diseases. Progress in Neurobiology, 162, 37–69. https://doi.org/10.1016/j.pneurobio.2017.12.003
- Popov, A., Brazhe, N., Fedotova, A., Tiaglik, A., Bychkov, M., Morozova, K., Brazhe, A., Aronov, D., Lyukmanova, E., Lazareva, N., Li, L., Ponimaskin, E., Verkhratsky, A., & Semyanov, A. (2022). A high-fat diet changes astrocytic metabolism to promote synaptic plasticity and behavior. Acta Physiologica, 236(1), e13847. https://doi.org/10.1111/apha.13847
- Sidoryk-Wegrzynowicz, M., Wegrzynowicz, M., Lee, E., Bowman, A. B., & Aschner, M. (2011). Role of Astrocytes in Brain Function and Disease. Toxicologic Pathology, 39(1), 115–123. https://doi.org/10.1177/0192623310385254